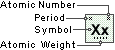Plutonium
Symbol
Pu
Atomic Number
94
Atomic Weight
244.0642
Element Classification
Discovered By
G.T.Seaborg, J.W.Kennedy, E.M.McMillan, A.C.Wohl
Discovery Date
1940 (United States)
Name Origin
Named for the planet Pluto.
Density (g/cc)
19.84
Melting Point (K)
914
Boiling Point (K)
3505
Appearance
Silvery-white, radioactive metal
Atomic Radius (pm)
151
Atomic Volume (cc/mol)
n/a
Covalent Radius (pm)
n/a
Ionic Radius
93 (+4e) 108 (+3e)
Specific Heat
n/a
Fusion Heat (kJ/mol)
2.8
Evaporation Heat (kJ/mol)
343.5
Thermal Conductivity (@25°C W/m K)
(6.7)
Debye Temperature (K)
n/a
Pauling Negativity Number
1.28
First Ionizing Energy (kJ/mol)
491.9
Oxidation States
6, 5, 4, 3
Electronic Configuration
[Rn] 5f6 7s2
Lattice Structure
Monoclinic(MCL)
Lattice Constant
n/a
Lattice C/A Ratio
n/a