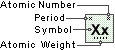Curium
Symbol
Cm
Atomic Number
96
Atomic Weight
247.0703
Element Classification
Discovered By
G.T.Seaborg, R.A.James, A.Ghiorso
Discovery Date
1944 (United States)
Name Origin
Named in honor of Pierre and Marie Curie.
Density (g/cc)
13.51
Melting Point (K)
1340
Boiling Point (K)
n/a
Appearance
Silvery, malleable, synthetic radioactive metal
Atomic Radius (pm)
299
Atomic Volume (cc/mol)
18.28
Covalent Radius (pm)
n/a
Ionic Radius
n/a
Specific Heat
n/a
Fusion Heat (kJ/mol)
n/a
Evaporation Heat (kJ/mol)
n/a
Thermal Conductivity (@25°C W/m K)
n/a
Debye Temperature (K)
n/a
Pauling Negativity Number
1.3
First Ionizing Energy (kJ/mol)
(580)
Oxidation States
4, 3
Electronic Configuration
[Rn] 5f7 6d1 7s2
Lattice Structure
(n/a)
Lattice Constant
n/a
Lattice C/A Ratio
n/a