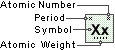Xenon
Symbol
Xe
Atomic Number
54
Atomic Weight
131.29
Element Classification
Discovered By
Sir William Ramsay; M. W. Travers
Discovery Date
1898 (England)
Name Origin
Greek: xenos (strange).
Density (g/cc)
3.52 (@ -109°C)
Melting Point (K)
161.3
Boiling Point (K)
166.1
Appearance
Heavy, colorless, and odorless noble gas
Atomic Radius (pm)
n/a
Atomic Volume (cc/mol)
42.9
Covalent Radius (pm)
131
Ionic Radius
n/a
Specific Heat
0.158
Fusion Heat (kJ/mol)
n/a
Evaporation Heat (kJ/mol)
12.65
Thermal Conductivity (@25°C W/m K)
0.0057
Debye Temperature (K)
n/a
Pauling Negativity Number
0.0
First Ionizing Energy (kJ/mol)
1170.0
Oxidation States
7
Electronic Configuration
[Kr] 4d10 5s2 5p6
Lattice Structure
Face-Centered Cubic(FCC)
Lattice Constant
6.200
Lattice C/A Ratio
n/a