Chemistry: What's an Ionic Compound?
What's an Ionic Compound?
We talked about the octet rule, which states that "all elements tend to want to gain or lose electrons so they have the same electron configuration as the nearest noble gas." Basically, what this means is that a neutral atom of any element other than a noble gas isn't very stable. As a result, it will gain or lose electrons until it attains the stable electron configuration of a noble gas. Atoms that have gained electrons are called anions and have negative charge, and atoms that have lost electrons are called cations and have positive charge. When anions stick to cations, the resulting chemical compound is called an ionic compound.
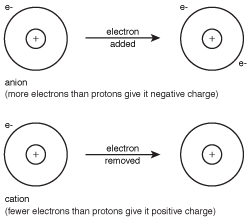
Figure 8.1Anions and cations are formed when an atom gains or loses electrons to achieve the electron configuration of the closest noble gas.
One question you may be asking yourself is, "How many electrons do different elements want to gain or lose?" The answer to this question, like many things in chemistry, can be found on the periodic table.
Molecular Meanings
An anion is a negatively charged atom or group of atoms, while a cation is an atom or group of atoms with positive charge. When an anion sticks to a cation, the result is an ionic compound.
The best way to figure out how many electrons will be gained or lost by a specific element is to count forward from it in the periodic table until you reach the next noble gas, and then count backward from it in the periodic table until you reach the last noble gas. If the forward direction requires less counting than the backward direction, the element will gain the same number of electrons as you counted to the closest noble gas to form an anion. Likewise, if the backward direction requires less counting, the element will lose the same number of electrons as you counted to the nearest noble gas to form a cation. Now, this is very important: Skip over the transition metals when counting to the noble gases—otherwise, things won't work out the way you'd like.
You've Got Problems
Problem 1: What will the charges of the following elements be when they gain or lose electrons to gain the same electron configurations as the nearest noble gas?
(a) magnesium (Mg)
(b) aluminum (Al)
(c) bromine (Br)
For example, oxygen forms ionic compounds as an anion with -2 charge because it needs to gain two electrons to have the same electron configuration as neon. Gallium, on the other hand, has a +3 charge because it needs to lose three electrons to have the same electron configuration as argon. If you're confused because it looks like you need to count backward by 13 rather than three, remember the rule to ignore the transition metals!
Excerpted from The Complete Idiot's Guide to Chemistry © 2003 by Ian Guch. All rights reserved including the right of reproduction in whole or in part in any form. Used by arrangement with Alpha Books, a member of Penguin Group (USA) Inc.
To order this book direct from the publisher, visit the Penguin USA website or call 1-800-253-6476. You can also purchase this book at Amazon.com and Barnes & Noble.







